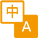Hieff Trans®脂质体核酸转染试剂是一种多用途的脂质体转染试剂,适用于DNA、RNA和寡核苷酸的转染,对大多数真核细胞具有很高的转染效率。其独特的配方使其可直接加入培养基中,血清的存在不会影响转染效率,这样可以减少去除血清对细胞的损伤。转染后不需要除去核酸-Hieff Trans®复合物或更换新鲜培养基,也可在4~6小时后除去。
Hieff Trans®脂质体核酸转染试剂以无菌的液体形式提供。通常情况下对于 24 孔板转染,每次用1.5 μL左右,则1 mL 约可做660 次转染;对于6孔板,每次用6 μL左右,则1 mL约可做160 次转染。
高效性: 可对大多数真核细胞进行转染,并且对于常见细胞系转染效率达90%以上;
低毒性:转染细胞形态良好并表达大量的转染基因蛋白;
操作简便:可以直接将脂质体复合物加入到含血清的培养基中;
多样性:适合瞬时转染和稳定转染试验。
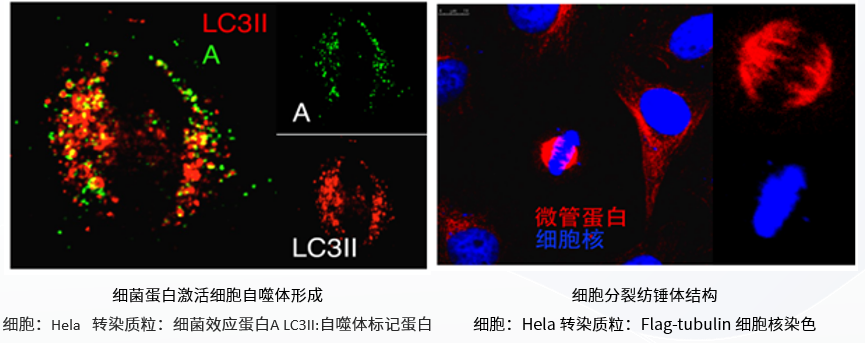
已验证细胞系
冰袋(wet ice)运输。产品2-8ºC保存,一年有效。不可冷冻!
[1] Liu R, Yang J, Yao J, et al. Optogenetic control of RNA function and metabolism using engineered light-switchable RNA-binding proteins. Nat Biotechnol. 2022;40(5):779-786. doi:10.1038/s41587-021-01112-1(IF:54.908)
[2] Fan Y, Wang J, Jin W, et al. CircNR3C2 promotes HRD1-mediated tumor-suppressive effect via sponging miR-513a-3p in triple-negative breast cancer. Mol Cancer. 2021;20(1):25. Published 2021 Feb 2. doi:10.1186/s12943-021-01321-x(IF:27.401)
[3] Tao R, Zhao Y, Chu H, et al. Genetically encoded fluorescent sensors reveal dynamic regulation of NADPH metabolism. Nat Methods. 2017;14(7):720-728. doi:10.1038/nmeth.4306(IF:25.062)
[4] Zhang Q, He X, Yao S, et al. Ablation of Mto1 in zebrafish exhibited hypertrophic cardiomyopathy manifested by mitochondrion RNA maturation deficiency. Nucleic Acids Res. 2021;49(8):4689-4704. doi:10.1093/nar/gkab228(IF:16.971)
[5] Liang Y, Lu Q, Li W, et al. Reactivation of tumour suppressor in breast cancer by enhancer switching through NamiRNA network. Nucleic Acids Res. 2021;49(15):8556-8572. doi:10.1093/nar/gkab626(IF:16.971)
[6] Wu S, Cao R, Tao B, et al. Pyruvate Facilitates FACT-Mediated γH2AX Loading to Chromatin and Promotes the Radiation Resistance of Glioblastoma. Adv Sci (Weinh). 2022;9(8):e2104055. doi:10.1002/advs.202104055(IF:16.806)
[7] Luo Q, Wu X, Zhao P, et al. OTUD1 Activates Caspase-Independent and Caspase-Dependent Apoptosis by Promoting AIF Nuclear Translocation and MCL1 Degradation. Adv Sci (Weinh). 2021;8(8):2002874. Published 2021 Feb 8. doi:10.1002/advs.202002874(IF:16.806)
[8] Chen S, Cao X, Zhang J, Wu W, Zhang B, Zhao F. circVAMP3 Drives CAPRIN1 Phase Separation and Inhibits Hepatocellular Carcinoma by Suppressing c-Myc Translation. Adv Sci (Weinh). 2022;9(8):e2103817. doi:10.1002/advs.202103817(IF:16.806)
[9] Yan JM, Zhang WK, Yan LN, Jiao YJ, Zhou CM, Yu XJ. Bunyavirus SFTSV exploits autophagic flux for viral assembly and egress. Autophagy. 2022;18(7):1599-1612. doi:10.1080/15548627.2021.1994296(IF:16.016)
[10] Xu X, Zhang J, Tian Y, et al. CircRNA inhibits DNA damage repair by interacting with host gene. Mol Cancer. 2020;19(1):128. Published 2020 Aug 24. doi:10.1186/s12943-020-01246-x(IF:15.302)
[11] Huang K, Chen X, Li C, et al. Structure-based investigation of fluorogenic Pepper aptamer. Nat Chem Biol. 2021;17(12):1289-1295. doi:10.1038/s41589-021-00884-6(IF:15.040)
[12] Li T, Chen X, Qian Y, et al. A synthetic BRET-based optogenetic device for pulsatile transgene expression enabling glucose homeostasis in mice. Nat Commun. 2021;12(1):615. Published 2021 Jan 27. doi:10.1038/s41467-021-20913-1(IF:14.919)
[13] Liu Z, Chen S, Lai L, Li Z. Inhibition of base editors with anti-deaminases derived from viruses. Nat Commun. 2022;13(1):597. Published 2022 Feb 1. doi:10.1038/s41467-022-28300-0(IF:14.919)
[14] Wu C, Wang C, Zheng J, et al. Vacuolization in Cytoplasm and Cell Membrane Permeability Enhancement Triggered by Micrometer-Sized Graphene Oxide. ACS Nano. 2015;9(8):7913-7924. doi:10.1021/acsnano.5b01685(IF:12.881)
[15] Zou Y, Wang A, Shi M, et al. Analysis of redox landscapes and dynamics in living cells and in vivo using genetically encoded fluorescent sensors. Nat Protoc. 2018;13(10):2362-2386. doi:10.1038/s41596-018-0042-5(IF:12.423)
[16] Sun X, Peng X, Cao Y, Zhou Y, Sun Y. ADNP promotes neural differentiation by modulating Wnt/β-catenin signaling. Nat Commun. 2020;11(1):2984. Published 2020 Jun 12. doi:10.1038/s41467-020-16799-0(IF:12.121)
[17] Song L, Liu Z, Hu HH, et al. Proto-oncogene Src links lipogenesis via lipin-1 to breast cancer malignancy. Nat Commun. 2020;11(1):5842. Published 2020 Nov 17. doi:10.1038/s41467-020-19694-w(IF:12.121)
[18] Shui S, Zhao Z, Wang H, Conrad M, Liu G. Non-enzymatic lipid peroxidation initiated by photodynamic therapy drives a distinct ferroptosis-like cell death pathway. Redox Biol. 2021;45:102056. doi:10.1016/j.redox.2021.102056(IF:11.799)
[19] Du L, Xie Y, Zheng K, et al. Oxidative stress transforms 3CLpro into an insoluble and more active form to promote SARS-CoV-2 replication [published online ahead of print, 2021 Nov 26]. Redox Biol. 2021;48:102199. doi:10.1016/j.redox.2021.102199(IF:11.799)
[20] Cen M, Ouyang W, Zhang W, et al. MitoQ protects against hyperpermeability of endothelium barrier in acute lung injury via a Nrf2-dependent mechanism. Redox Biol. 2021;41:101936. doi:10.1016/j.redox.2021.101936(IF:11.799)
[21] Sun X, Peng X, Cao Y, Zhou Y, Sun Y. ADNP promotes neural differentiation by modulating Wnt/β-catenin signaling. Nat Commun. 2020;11(1):2984. Published 2020 Jun 12. doi:10.1038/s41467-020-16799-0(IF:11.614)
[22] Liu W, Zhan Z, Zhang M, et al. KAT6A, a novel regulator of β-catenin, promotes tumorigenicity and chemoresistance in ovarian cancer by acetylating COP1. Theranostics. 2021;11(13):6278-6292. Published 2021 Apr 15. doi:10.7150/thno.57455(IF:11.556)
[23] Hao Q, Li J, Zhang Q, et al. Single-cell transcriptomes reveal heterogeneity of high-grade serous ovarian carcinoma. Clin Transl Med. 2021;11(8):e500. doi:10.1002/ctm2.500(IF:11.492)
[24] Zhang Y, Yu X, Sun R, et al. Splicing factor arginine/serine-rich 8 promotes multiple myeloma malignancy and bone lesion through alternative splicing of CACYBP and exosome-based cellular communication. Clin Transl Med. 2022;12(2):e684. doi:10.1002/ctm2.684(IF:11.492)
[25] Liu Z, Chen S, Xie W, et al. Versatile and efficient in vivo genome editing with compact Streptococcus pasteurianus Cas9. Mol Ther. 2022;30(1):256-267. doi:10.1016/j.ymthe.2021.06.013(IF:11.454)
[26] Tang X, Deng Z, Ding P, et al. A novel protein encoded by circHNRNPU promotes multiple myeloma progression by regulating the bone marrow microenvironment and alternative splicing. J Exp Clin Cancer Res. 2022;41(1):85. Published 2022 Mar 8. doi:10.1186/s13046-022-02276-7(IF:11.161)
[27] Gu C, Wang Y, Zhang L, et al. AHSA1 is a promising therapeutic target for cellular proliferation and proteasome inhibitor resistance in multiple myeloma. J Exp Clin Cancer Res. 2022;41(1):11. Published 2022 Jan 6. doi:10.1186/s13046-021-02220-1(IF:11.161)
[28] Chen P, Zhou J, Wan Y, et al. A Cas12a ortholog with stringent PAM recognition followed by low off-target editing rates for genome editing. Genome Biol. 2020;21(1):78. Published 2020 Mar 25. doi:10.1186/s13059-020-01989-2(IF:10.806)