DiO是一种亲脂性的荧光染料,可以用来染细胞膜和其它脂溶性生物结构。进入细胞膜后,DiO在整个细胞膜上扩散,最佳浓度时可以使整个细胞膜染色。DiO在进入细胞膜之前荧光非常弱,当与细胞膜结合后其荧光强度大大增强,被激发后可以发出绿色的荧光,具有很高的淬灭常数和激发态寿命。可以用标准的FITC滤光片检测。
DiO通常不会明显影响细胞的生存力,因此被广泛用于正向或逆向的,活的或固定的神经等细胞或组织的示踪剂或长期示踪剂(long-term tracer)。除了最简单的细胞膜荧光标记外,还可以用于检测细胞的融合和粘附,检测发育或移植过程中细胞迁移,通过FRAP(Fluorescence Recovery After Photobleaching)检测脂在细胞膜上的扩散,检测细胞毒性和标记脂蛋白等。
| 同义名(Synonym) | DiOC18(3); DiO perchlorate; 3,3-Dioctadecyloxacarbocyanine perchlorate |
| CAS 号(CAS NO.) | 34215-57-1 |
| 分子式(Molecular Fomular) | C53H85ClN2O6 |
| 分子量(Molecular Weight) | 881.72 |
| 外观(Appearance) | 橘色至红色固体 |
| Ex/Em | 484/501 nm |
| 推荐滤光器(Filter) | XF23-Omega, 31001-Chroma |
| 纯度(Purity) | >97% |
| 溶解性(Solubility) | 溶于DMF,DMSO,乙醇 |
| 结构式(Structure) | 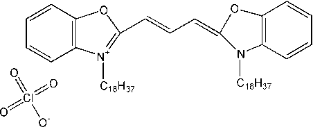 |
冰袋运输。产品-20 ºC干燥避光保存,有效期1年。
[1] Ma W, Zhang X, Liu Y, et al. Polydopamine Decorated Microneedles with Fe-MSC-Derived Nanovesicles Encapsulation for Wound Healing. Adv Sci (Weinh). 2022;9(13):e2103317. doi:10.1002/advs.202103317(IF:16.806)
[2] Duan J, Bao C, Xie Y, et al. Targeted core-shell nanoparticles for precise CTCF gene insert in treatment of metastatic breast cancer. Bioact Mater. 2021;11:1-14. Published 2021 Oct 7. doi:10.1016/j.bioactmat.2021.10.007(IF:14.593)
[3] Wang D, Dong H, Li M, et al. Erythrocyte-Cancer Hybrid Membrane Camouflaged Hollow Copper Sulfide Nanoparticles for Prolonged Circulation Life and Homotypic-Targeting Photothermal/Chemotherapy of Melanoma. ACS Nano. 2018;12(6):5241-5252. doi:10.1021/acsnano.7b08355(IF:13.709)
[4] Yao C, Zhang R, Tang J, Yang D. Rolling circle amplification (RCA)-based DNA hydrogel. Nat Protoc. 2021;16(12):5460-5483. doi:10.1038/s41596-021-00621-2(IF:13.491)
[5] Zhang Z, Liu Q, Tan J, et al. Coating with flexible DNA network enhanced T-cell activation and tumor killing for adoptive cell therapy. Acta Pharm Sin B. 2021;11(7):1965-1977. doi:10.1016/j.apsb.2021.04.002(IF:11.614)
[6] Liu Z, Zhu Q, Song E, Song Y. Characterization of blood protein adsorption on PM2.5 and its implications on cellular uptake and cytotoxicity of PM2.5. J Hazard Mater. 2021;414:125499. doi:10.1016/j.jhazmat.2021.125499(IF:10.588)
[7] Zhou H, You P, Liu H, et al. Artemisinin and Procyanidins loaded multifunctional nanocomplexes alleviate atherosclerosis via simultaneously modulating lipid influx and cholesterol efflux. J Control Release. 2022;341:828-843. doi:10.1016/j.jconrel.2021.12.021(IF:9.776)
[8] Xu C, Liu W, Hu Y, Li W, Di W. Bioinspired tumor-homing nanoplatform for co-delivery of paclitaxel and siRNA-E7 to HPV-related cervical malignancies for synergistic therapy. Theranostics. 2020;10(7):3325-3339. Published 2020 Feb 10. doi:10.7150/thno.41228(IF:8.579)
[9] Zhang Q, Yi DY, Xue BZ, et al. CD90 determined two subpopulations of glioma-associated mesenchymal stem cells with different roles in tumour progression. Cell Death Dis. 2018;9(11):1101. Published 2018 Oct 27. doi:10.1038/s41419-018-1140-6(IF:8.469)
[10] Xie Q, Liu Y, Long Y, et al. Hybrid-cell membrane-coated nanocomplex-loaded chikusetsusaponin IVa methyl ester for a combinational therapy against breast cancer assisted by Ce6. Biomater Sci. 2021;9(8):2991-3004. doi:10.1039/d0bm02211j(IF:6.843)
[11] Li X, Yang Z, Nie W, et al. Exosomes derived from cardiac progenitor cells attenuate CVB3-induced apoptosis via abrogating the proliferation of CVB3 and modulating the mTOR signaling pathways. Cell Death Dis. 2019;10(10):691. Published 2019 Sep 18. doi:10.1038/s41419-019-1910-9(IF:5.959)
[12] Yi D, Xiang W, Zhang Q, et al. Human Glioblastoma-Derived Mesenchymal Stem Cell to Pericytes Transition and Angiogenic Capacity in Glioblastoma Microenvironment. Cell Physiol Biochem. 2018;46(1):279-290. doi:10.1159/000488429(IF:5.500)
[13] Zhang Y, Guo S, Huang H, Mao G, Ji X, He Z. Silicon nanodot-based aptasensor for fluorescence turn-on detection of mucin 1 and targeted cancer cell imaging. Anal Chim Acta. 2018;1035:154-160. doi:10.1016/j.aca.2018.06.032(IF:5.123)
[14] Li H, Hu Y, Zeng M, et al. Exosomes From Human Urine-Derived Stem Cells Encapsulated Into PLGA Nanoparticles for Therapy in Mice With Particulate Polyethylene-Induced Osteolysis. Front Med (Lausanne). 2021;8:781449. Published 2021 Dec 6. doi:10.3389/fmed.2021.781449(IF:5.093)
[15] Ding T , Wang L , Zhang J , Xing Y , Cai K . Interfacially active polydopamine for nanoparticle stabilized nanocapsules in a one-pot assembly strategy toward efficient drug delivery. J Mater Chem B. 2018;6(12):1754-1763. doi:10.1039/c7tb03008h(IF:4.776)
[16] Xie XL, Wei Y, Song YY, et al. Genetic Analysis of Four Sexual Differentiation Process Proteins (isp4/SDPs) in Chaetomium thermophilum and Thermomyces lanuginosus Reveals Their Distinct Roles in Development. Front Microbiol. 2020;10:2994. Published 2020 Jan 6. doi:10.3389/fmicb.2019.02994(IF:4.236)
[17] Zhou W, Zhao Z, Yu Z, Hou Y, Keerthiga R, Fu A. Mitochondrial transplantation therapy inhibits the proliferation of malignant hepatocellular carcinoma and its mechanism. Mitochondrion. 2022;65:11-22. doi:10.1016/j.mito.2022.04.004(IF:4.160)
[18] Wang YY, Xia K, Wang ZX, Xie H, Xu R. Osteocyte exosomes accelerate benign prostatic hyperplasia development. Mol Cell Endocrinol. 2021;531:111301. doi:10.1016/j.mce.2021.111301(IF:4.102)
[19] Wang S, Li Y, Zhao Y, Lin F, Qu J, Liu L. Investigating tunneling nanotubes in ovarian cancer based on two-photon excitation FLIM-FRET. Biomed Opt Express. 2021;12(4):1962-1973. Published 2021 Mar 9. doi:10.1364/BOE.418778(IF:3.732)
[20] Zhu X, Qin X, Wang X, et al. Oral cancer cell‑derived exosomes modulate natural killer cell activity by regulating the receptors on these cells. Int J Mol Med. 2020;46(6):2115-2125. doi:10.3892/ijmm.2020.4736(IF:3.098)
[21] Li S, Jiang J, Yang Z, Li Z, Ma X, Li X. Cardiac progenitor cell‑derived exosomes promote H9C2 cell growth via Akt/mTOR activation. Int J Mol Med. 2018;42(3):1517-1525. doi:10.3892/ijmm.2018.3699(IF:2.784)